
by Improov
In recent years, the concept of quality monitoring for laboratory testing has evolved beyond the analytic phase of testing to encompass the total testing process. Beginning with test ordering and ending with result reporting, this concept encompasses the...

by Improov
Whether planning for a new laboratory, or analyzing the appropriateness of your current testing, it is important to do a periodic assessment not only of what you want to offer your ordering physicians, but what you can realistically offer. The laboratory’s test menu...
by Improov
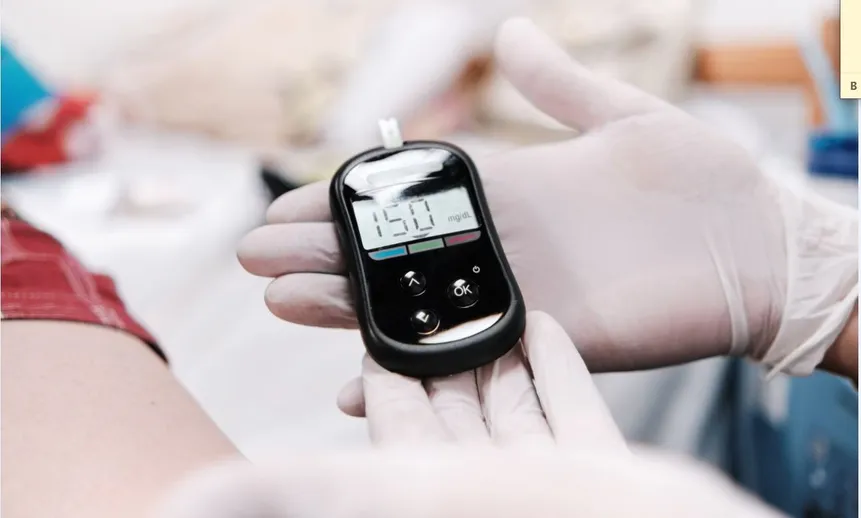
Management of POCT can be challenging. Testing may include multiple sites, many different POCT devices/kits, and dozens of operators that have to be managed in order to assure quality patient care. It is therefore important to have a robust POCT management system;...

by Improov
One of the fastest areas of growth in laboratory medicine is point-of-care testing (POCT), with estimated global expenditures exceeding $24.2 billion in 2019 and growth (pre-COVID19 Pandemic estimates) projected at over 7.1% annually between 2020 and 2026. The COVID19...

by Improov
Hello! Welcome to the Improov Weekly Blog where we will discuss all issues related to the laboratory profession, from initiating testing to accreditation preparation; from automation to customer service; from personnel competency to inventory control; from quality...






