In recent years, the concept of quality monitoring for laboratory testing has evolved beyond the analytic phase of testing to encompass the total testing process. Beginning with test ordering and ending with result reporting, this concept encompasses the pre-analytical, analytical, and post-analytical phases of testing. The impetus for total testing process (TTP) quality monitoring has been the realization that errors occur throughout all phases of testing, not just during the analytic phase.
In fact, “up to 70% of all errors made in laboratory testing occurs during the pre-analytic phase”[i] This phase encompasses patient preparation, specimen collection, transport, storage, and processing, as well as the maintenance of adequate inventory and proper storage conditions for reagents and related testing supplies.
An essential requirement for quality testing is to maintain the integrity of patient samples, reagents, and the test systems utilized. This is best achieved by the laboratory having a reliable system that monitors these items, maintains complete records and documentation, as well as meeting all regulatory and institutional requirements[ii].
Protecting Test Specimen Integrity
Since specimen integrity is dependent upon proper performance of pre-analytical processes, compromises to specimen integrity can give erroneous results and compromise the care of the patient. The following guidelines cover some of the key steps in handling patient samples to provide optimal specimens for testing:
Patient Preparation[iii]
Ensuring proper patient preparation is one of the most challenging requirements of the pre-analytical phase because it encompasses activities that typically occur before the individual arrives for his or her sample collection. Patient preparation factors include:
Diet:
Food consumption is a significant source of pre-analytical variability. This effect varies based on the analyte(s) to be measured, and the time between consumption and sample collection. An overnight fasting period of 10 to 14 hours prior to collection is optimal for minimizing variations. However, some foods may take longer to metabolize, and particular foods should be prohibited before performing certain tests. Communicating these requirements to patients is important to ensure appropriate preparation for testing.
Timing of Sample Collection
Blood concentrations of various analytes change during the course of the day. These cyclical variations can be significant, so the timing of sample collection should be strictly controlled. The timing of sample collection is especially critical for therapeutic drug monitoring, which requires trough levels for most analytes. Protocols must specify an ideal time of sampling for each test and the actual time of draw must be carefully documented.
Specimen Collection[iv]
Specimen collection poses several opportunities for errors. Important factors to consider for reducing variability during this phase of testing include positive patient identification, correct collection tube type and order of draw, and proper sample volume and mixing.
Patient Identification
Improper patient identification is a major concern due to the possible severe consequences from mislabeling a specimen. When collecting samples, two unique identifiers are required for positive identification. When implemented, wristband barcodes, and barcode scanners have significantly reduced rates of misidentification.
Collection Tubes
Several types of tubes are available for specimen collection depending on the different additives and barriers used for distinct applications. For example, additives may promote or inhibit clotting to produce serum or plasma, respectively. As a result, the order of collecting multiple tubes through the same needle is important, and should proceed from tubes with no additives to tubes with very strong additives.
Specimen Volume and Proper Tube Mixing:
All sample collection tubes need to be filled with the appropriate volume. This ensures the proper amount of specimen to amount of additive in the tube. In addition, all tubes with additives need to be gently inverted to mix the additive evenly with the blood. Excessive shaking will rupture cells and cause hemolysis.
Specimen Processing, Transport and Storage[v]
Transport can be a significant source of specimen problems. The main variables to consider include agitation, light exposure, temperature, transport time, and placement of samples within the proper transport container. Specimens should be delivered to the laboratory promptly after collection, and the time between sampling and analysis reduced to a minimum. In addition, samples should be transported and stored under proper temperature and light conditions.
The time between collection and centrifugation affects some analytes more than others. When plasma or serum are required, labs should centrifuge samples (and aliquot, if necessary) prior to transportation if the sample is traveling > 1-2 hours to the central lab. Labs need standardized protocols for centrifugation time and speed, because these variables impact specimen integrity. Re-centrifugation should be avoided because it can cause hemolysis and affects gel-barrier integrity.
Specimen collection and handling factors that can affect specimen integrity[vi]:
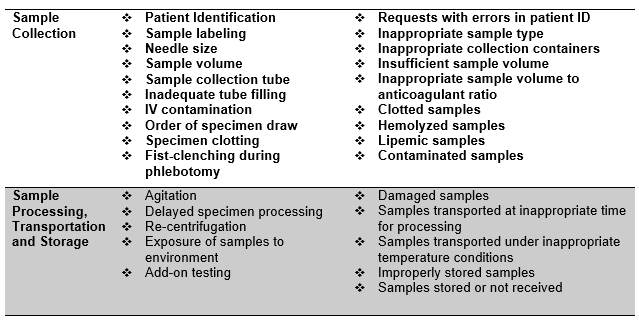
Personnel Training and Competency[vii]
Unlike the analytic phase, the processes of the pre-analytic phase, including test ordering, patient preparation, specimen collection, identification, handling, and transport, often involve personnel that are not under the direct supervision of the laboratory, such as phlebotomists, nurses, administrative personnel, even receptionists and other office staff. This makes it more challenging to control quality performance and address errors[viii].
Proper training of personnel who collect and handle specimens is the key to ensuring sample integrity. Improper training can lead to high sample rejection volume and/or compromised patient results, which can affect the outcome of patient care. Thus, all personnel involved with pre-laboratory as well as laboratory-centered pre-analytical processes must have their training documented and their competency evaluated by qualified laboratory professionals.
Be Proactive to Ensure Sample Integrity:[ix]
-
Properly train and perform competency assessments on all personnel that collect patient samples.
-
When collecting samples, ensure that the proper container is used. If in doubt, consult the laboratory manual or call the laboratory.
-
Provide a detailed specimen collection procedure to all sites that submit samples to the laboratory. Include a section for rejecting specimens.
-
Properly store all samples per manufacturer’s recommendations. If the laboratory performs laboratory-developed tests (LDT), specimen stability studies for specimen age, storage and transport must be performed.
-
Ensure that all samples contain at least two unique identifiers and the information on the sample matches the information on the requisition.
[i]Quality Indicators To Detect Pre-Analytical Errors In Laboratory Testing. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3428256/
[ii] Safeguard Specimen Integrity With Real-Time Laboratory Monitoring. https://blog.xiltrixusa.com/safeguard-specimen-integrity-with-real-time-laboratory-monitoring
[iii] Ghaedi, M., El-Khoury, J. Pre-Analytical Variation. The Leading Cause of Error in Laboratory Medicine. Clinical laboratory News (CLN) July 1, 2016. https://www.aacc.org/publications/cln/articles/2016/july/preanalytical-variation-the-leading-cause-of-error-in-laboratory-medicine
[iv] Ibid.
[v] Ibid.
[vi] Ibid.
[vii]Safeguard Specimen Integrity With Real-Time Laboratory Monitoring. https://blog.xiltrixusa.com/safeguard-specimen-integrity-with-real-time-laboratory-monitoring
[viii] Lippi, G., Banfi, G., Church, S., Cornes, M., De Carki, G., et al. Opinion Paper on behalf of the European Federation for Clinical Chemistry and Laboratory Medicine Working Group for Preanalytical Phase. Clin Chem Lab Med. October 26, 2014. https://www.eflm.eu/files/efcc/4.6%20Lippi%20CCLM-2014-1051.pdf
[ix] Things You Can Do To Insure Specimen Integrity. COLA.org. Educational Resources. Patient Safety Program. https://www.cola.org/education-resources/free-resources/patient-safety/
